.Flick the tubes gently to mix—do not vortex. Centrifuge the tubes briefly (10 sec. at max rpm) and return to ice immediately.
C. Bringing the termination mixes and DNA Master Mixes together (Estimated Time 15-30 minutes)
4. Label four 0.2 mL PCR tubes for each DNA Master Mix “A, C, T, and G”.
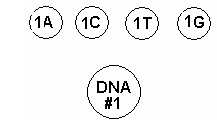
9.Aliquot 6 µL of the Master Mix from section B into each of the 0.2 mL PCR tubes you have just labeled, A, C, T, and G. Return tubes to ice.
10.Aliquot 2 µL of the corresponding termination mix ddA, ddC, ddG, ddT from section A into each A, C, T, G labeled 0.2 ml PCR tube. Return tubes to ice
11.Flick the tubes gently to mix—do not vortex. Centrifuge the tubes briefly (10 sec. at max rpm) to collect at the bottom of the tube and return to ice immediately.
D. Programming the Thermalcycler (Estimated Time 15-25 minutes for set up and 2.5 -3.0 hours for cycling)
12.Program and run the thermocycler (PCR machine) using the following parameters.
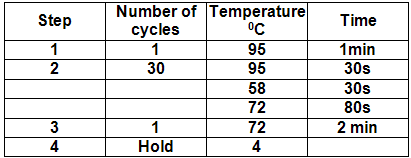
**It is a good idea to test the program parameters before beginning the run.
E. Preparation for Sequencing
NOTE: Unincorporated dye terminators (ddNTPs) can be removed from the sequencing reactions by ethanol precipitation of the DNA.
13.Centrifuge the tubes briefly (10 sec. at max rpm) to collect at the bottom of the tubes.
14.Transfer the PCR reactions into labeled 1.5 microfuge tubes. *There will be one 1.5 mL microfuge tube for every PCR tube. Be careful and descriptive with labeling and transferring.
15.To each labeled 1.5 mL microfuge tube add:
2µL of 7.5mM Ammonium acetate (provided by TA’s)
2µL Glycogen solution (provided with Sequencing Kit)
30µL (3x reaction volume) of chilled 100% ethanol (provided by TA’s)
Mix thoroughly by flicking the tubes, centrifuge the tubes briefly (10 sec. at max rpm) to collect at the bottom of the tubes and place in -20°C for 20-45 minutes to precipitate the DNA.
16.Centrifuge all tubes at full speed (room temperature) in a table-top microcentrifuge for 20-30min to pellet the precipitated DNA.
17. Carefully remove all supernatant with a 10 µL pipet . (A large tip can cause damage to your DNA). All ethanol must be removed from the tube!
18. Carefully add 200 µL of chilled 70% ethanol to the tube and briefly flick the tube to resuspend the pellet. (Pellets are very fragile at this point and are clear—may be hard to see.) Centrifuge at full speed for 5 minutes. This provides and additional wash.
19. Carefully remove the supernatants with 10 µL pipet and air dry the pellet on the table top for 5 minutes by leaving the lid on the tube open. If pellets are overdried, they may be difficult to resuspend.
20.Resuspend each pellet in 6 µL of Formamide (provided with Sequencing Kit) loading dye and vortex vigorously (10-20 sec). Briefly centrifuge to collect sample at the bottom of the tube. Return samples to ice.
*The DNA pellets must be completely resuspended at this step for optimal sequencing results.
F. Sequencing
The ALFExpress equipment will be set up by the instructor or TA.
21.Just prior to loading the samples onto the gel, heat each sample to 70ºC in a water bath for 2-4 min to denature, and then cool immediately on ice.
22.Using the 100 uL flat tips, load the entire volume (6µL) of each sample into separate lanes of the sequencing gel.
After all samples have been loading the sequencing process will be initiated by the instructor or TA.
DNA sequencing is the determination of the precise sequence of nucleotides in a sample of DNA. The most popular method for doing this is called the dideoxy method.
The equipment and supplies:
·Sterile 1.5 mL and 0.2 mL microcentrifuge tubes and racks
·Ice
· Micropipettes, 0.5 to 1.0 µl, 10 to 100 µl, 100 to 1000 µl
·Sterile pipette tips
·Disposable gloves, safety glasses, and lab coats
·Microcentrifuge, for 1.5 ml and 0.2 ml PCR (adapter) microcentrifuge tubes
·Sterile deionized water
·Template DNA
·Primers (Always determine the concentration of primer stock by reading at 260 nm (OD260).
·PCR machine (Thermocycler)
·7.5 Molar Ammonium acetate aqueous solution
·Freezer, -20°C
·Water bath set at 70°C
·37°C incubator
·Sterile gel loading pipet tips
·UV-box for photo-polymerization
·Lint-free medical wipes (Kimwipes or similar)
·Thermo Sequenase Cy5 Dye Terminator Cycle Sequencing Kit Amersham Biosciences (#27-2682-01), store at -20ºC
·Alfexpress II DNA Sequencer from Amersham Biosciences
·Long Read Gel Kit
·TBE Buffer
Protocol
**Important notes before you begin: All reagents should be kept on ice when removed from storage for use. Also please be aware that repeated freeze-thawing of the Cy-labeled terminators is not recommended. All reagent tubes should be centrifuged before opening them. Whenever possible, keep the tubes capped and on ice to minimize evaporation of the small volumes of the reagents used. Thoroughly mix all reaction mixtures after each addition and centrifuge tubes briefly to collect the reaction mixtures at the bottom of the tubes. Dispense all reagents carefully with new tips for each transfer to avoid contamination of stock solutions.
***Safety Warnings and Precautions: All chemicals should be considered as potentially hazardous. Wear suitable protective clothing such as lab coat, safety glasses, and gloves. Care should be taken to avoid contact with skin or eyes. This protocol requires the use of ethanol and formamide. Gel reagents may contain acrylamide, a neurotoxin and suspected carcinogen. Please follow the manufacturer’s Material Safety Data Sheet regarding safe handling and use of these materials.
A. Preparation of ddA, ddC, ddG, ddT termination mixes (Estimated Time 15-30 min.)**Only one set of termination mixes are required to sequence up to 10 DNA templates—DO NOT set up termination mixes for each template!
1.Label four 1.5 mL microfuge tubes “ddA, ddC, ddG, ddT” for the respective termination mixes.
2.To each tube, add the components indicated below to prepare the dNTP/Cy5 ddNTP termination mixes, beginning with the water first. The volumes are sufficient for 10 DNA template sequencing reactions.
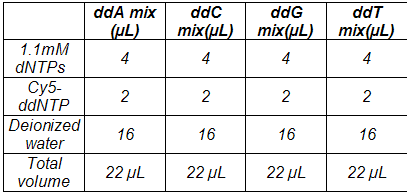
3.Flick the tubes gently to mix—do not vortex. Centrifuge the tubes briefly (10 sec. at max rpm) to collect the contents at the bottom of each tube.
4. Store the dNTP/ Cy5-ddNTP mixes on ice and in the dark until needed. (Cover tubes with aluminum foil).
B. Preparation of Master Mix (Estimated Time 15-30 minutes)
NOTE: Make sure you have a water bath heating to 70ºC while mixes are being prepared. It will need to be up to temperature prior to loading samples for sequencing.
5. Label one 1.5 mL microcentrifuge tube with the DNA sample name for each DNA template with primer to be sequenced. Keep tubes on ice and capped when not in use. Ex. “DNA#1 Forward Master Mix”, “DNA #1 Reverse Master Mix”, “DNA #2 Forward Master Mix”, etc.
6. Using the table below, each group will prepare master mixes for each DNA template with primer to be sequenced. Begin by adding the water first, Reaction Buffer, DNA and primer, and Thermo Sequenase polymerase last. **Important: The Thermo Sequenase polymerase must be kept on ice and capped whenever possible. Handling time must be kept brief.
|
DNA template (0.2-0.5 µg)
|
____ µL
|
|
Primer (4 pmol total)
|
2 µL
|
|
Reaction Buffer
|
4.0 µL
|
|
Thermo Sequenase I DNA polymerase (10 U/ul)
|
1.0 µL
|
|
Distilled Water (to a final volume of 27 µL)
|
____ µL
|
|
Total volume
|
27.0 µL
|
|